Velocity Clinical Research (“Velocity”), the leading multi-specialty clinical site organization, today announces it has made three C-suite hires, as the company continues to dramatically expand its clinical site and patient organization, bringing research opportunities to more people. Nick Campbell joins as Chief Commercial Officer, … Read more
To gain deeper insight into the perspectives of clinical trial participants, Velocity has built the Participant Advisory Committee (PAC). The PAC comprises diverse current and past clinical trial participants from across the country. The Velocity PAC convenes regularly to discuss issues and share ideas … Read more
Tyler Adam, MD, Principal Investigator at Velocity Clinical Research in Hastings, NE, supported the promising MATISSE Phase 3 maternal RSV vaccine study — results of which have been published in the article, “Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants” … Read more
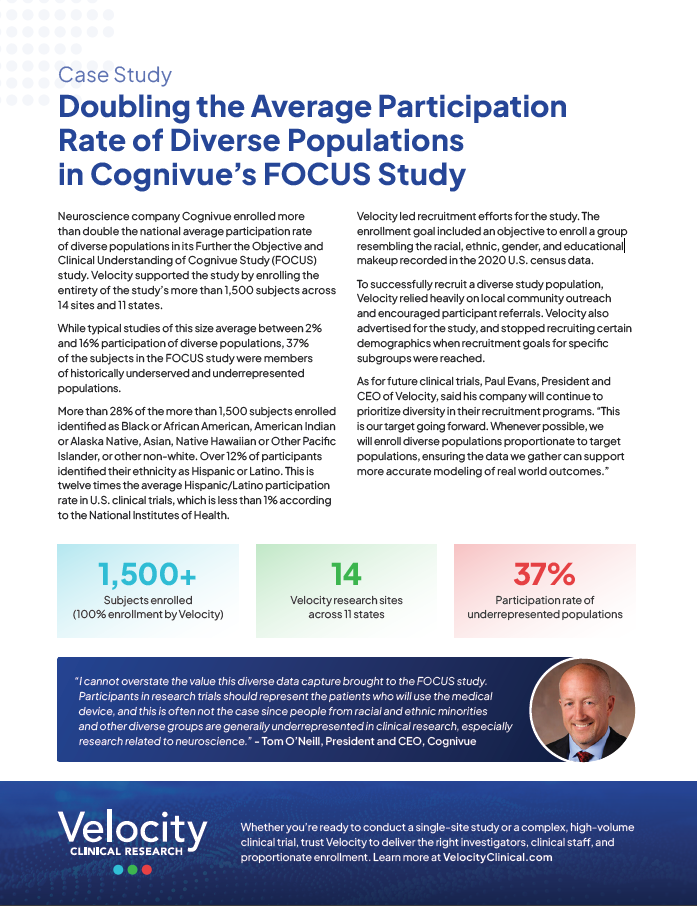
Case Study: Doubling the Average Participation Rate of Diverse Populations in Cognivue’s FOCUS Study
Neuroscience company Cognivue enrolled more than double the national average participation rate of diverse populations in its Further the Objective and Clinical Understanding of Cognivue Study (FOCUS) study. Velocity supported the study by enrolling the entirety of the study’s more than 1,500 subjects across … Read more
Velocity Clinical Research is now a Society for Clinical Research Sites (SCRS) Global Impact Partnership (GIP). Paul Evans, PhD, President and CEO of Velocity, said the company is excited to leverage this partnership to further improve clinical research, both within Velocity and industry-wide. “The … Read more
Clinical trials — especially high-volume trials — are primarily designed to collect quantitative data. Collecting and reviewing qualitative data and experiential feedback from trial participants is rarely prioritized. Before being acquired by Velocity Clinical Research, Meridian Clinical Research built an in-house surveying system to … Read more
Velocity Clinical Research (“Velocity”), the largest integrated research site organisation globally, today announces it has strengthened its European senior executive team with three strategic hires, as the firm continues its expansion in the region. Évelyne Newton joins as Vice President of Business Development, Sandra … Read more
1: Consolidation and scale of the market It is well known that fragmentation of the clinical sites business has been identified as a market opportunity by private equity firms. As a result of a PE capital injection, there has been increased merger and acquisition … Read more
Brandon Essink, MD, CPI, was the lead author of the article, “The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials”. Dr. Essink oversaw … Read more
DURHAM, N.C., July 27, 2022 /PRNewswire/ — Velocity Clinical Research (“Velocity”), the leading integrated research site organisation, today announces it has acquired its first European site, Clinical Research Hamburg, in Germany for an undisclosed amount. The acquisition is the first in a series of deals lined up across … Read more
Velocity Clinical Research (“Velocity”), the leading integrated research site organization, today announces its acquisition of Meridian Clinical Research for an undisclosed amount. The deal makes Velocity the largest dedicated research site organization in the world with approximately 80 sites in the U.S. and Europe, … Read more
Learn more about Velocity's capabilities
Whether you’re ready to conduct a single-site study or a complex, high-volume trial, Velocity will ensure you have the right investigators, clinical staff, and patients for your research program.
Join a remarkable team doing remarkable work
Velocity careers offer competitive pay and benefits, and reward high performance with excellent opportunities.
Whether you're an industry veteran or are looking to take your first step into clinical research, we invite you to apply at Velocity.