For the team at Velocity Clinical Research in the United Kingdom, clinical trials are first and foremost about building relationships. “I’m really grateful to be involved in clinical trials, because it allows me to develop meaningful connections with people in a way that goes beyond the traditional and [usually] more formal doctor-patient relationship,” says Ahmed … Read more
With nearly three decades as a family physician and almost 25 years as a Principal Investigator (PI), Frank Eder, MD, believes two primary elements are critical for sustained success in clinical research. “You must have a true interest in the research side of it. You have to actually be interested in the science and the … Read more
Julio Rosenstock, MD, and Juan Frias, MD, were featured prominently throughout the American Diabetes Association (ADA) 83rd Scientific Sessions. The prolific researchers also had findings simultaneously published in the New England Journal of Medicine and The Lancet. Dr. Rosenstock is the lead investigator for Novo Nordisk’s ONWARDS 1 program exploring the first investigational once-weekly insulin. … Read more
The Velocity Clinical Research team in Los Angeles, CA, is tackling two of the biggest health threats to Americans today: obesity and diabetes. Better still, they’re making tremendous progress against these conditions that exact a terrible toll on quality of life and longevity. Consider these sobering statistics from the Centers for Disease Control and Protection … Read more
Velocity Clinical Research maintains a Participant Advisory Committee (PAC) — a diverse mix of current and past trial participants across the U.S. The PAC’s job? Providing participant input to fine-tune the clinical research process. In this piece, we dig into highlights from PAC’s recent meetings in March, April, and May 2023. We’ll cover three key … Read more
In this ongoing series, Velocity puts the spotlight on innovative Principal Investigators (PIs) whose work is changing the medical landscape at the intersection of research and patient care. Velocity Team Contributes to Potential Breakthrough Sleep Apnea Treatment Enjoying a good night’s sleep is vitally important both mentally and physically. Unfortunately, for many it’s a struggle … Read more
To gain deeper insight into the perspectives of clinical trial participants, Velocity has built the Participant Advisory Committee (PAC). The PAC comprises diverse current and past clinical trial participants from across the country. The Velocity PAC convenes regularly to discuss issues and share ideas that could improve the clinical research process now and for future … Read more
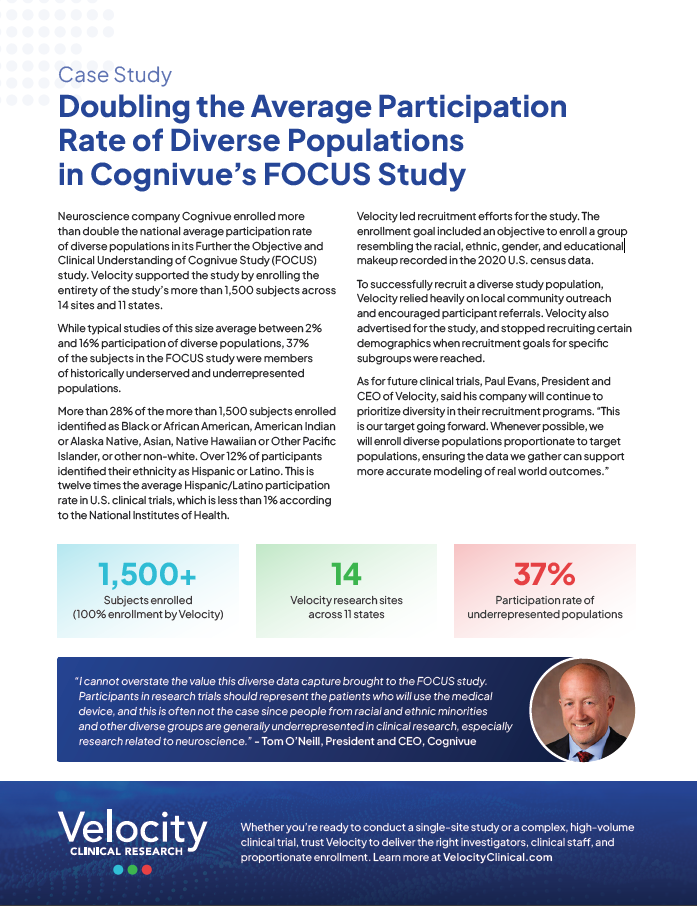
Case Study: Doubling the Average Participation Rate of Diverse Populations in Cognivue’s FOCUS Study
Neuroscience company Cognivue enrolled more than double the national average participation rate of diverse populations in its Further the Objective and Clinical Understanding of Cognivue Study (FOCUS) study. Velocity supported the study by enrolling the entirety of the study’s more than 1,500 subjects across 14 sites and 11 states. While typical studies of this size … Read more
Clinical trials — especially high-volume trials — are primarily designed to collect quantitative data. Collecting and reviewing qualitative data and experiential feedback from trial participants is rarely prioritized. Before being acquired by Velocity Clinical Research, Meridian Clinical Research built an in-house surveying system to elevate the participant’s voice. The system, which is largely automated, collects … Read more
- « Previous
- 1
- 2
- 3
- 4
Learn more about Velocity's capabilities
Whether you’re ready to conduct a single-site study or a complex, high-volume trial, Velocity will ensure you have the right investigators, clinical staff, and patients for your research program.
Join a remarkable team doing remarkable work
Velocity careers offer competitive pay and benefits, and reward high performance with excellent opportunities.
Whether you're an industry veteran or are looking to take your first step into clinical research, we invite you to apply at Velocity.