Even as a teen in Egypt, Amira Nada was drawn to the idea of clinical research and how it could help people live healthier, richer lives. “When I was a young girl, I saw people around me suffering from mental and other health challenges, and … Read more
Inc. magazine ranked Velocity Clinical Research 543 on its annual Inc. 5000 list, the most prestigious ranking of the nation’s fastest-growing private companies. Headquartered in Durham, NC, Velocity is the world’s leading integrated site organization with 80 sites worldwide. This is Velocity’s first recognition … Read more
More than ever, most of us have jobs that involve sitting at a desk for hours on end. The average desk worker in the U.S. is 36 years old and female. While some companies invest in workstation assessments, a lot of us are still … Read more
Velocity is collaborating with Merck to explore a new way to engage communities about clinical research. Starting this month the Velocity team members are conducting a tour of several Southern California cities, using Merck’s iLab53, a state-of-the-art, modular 39-foot mobile research unit to reach … Read more
At Velocity Clinical Research, we prioritize the voices of clinical trial participants, largely via the Participant Advisory Committee (PAC). Our PAC, which brings together a diverse group of past and current trial participants from across the U.S., offers a unique lens into the clinical … Read more
Velocity Clinical Research, the leading integrated site organization for clinical trials, has launched a groundbreaking initiative: Councils to Accelerate Research Excellence (CARE Councils). The CARE Councils underpin Velocity’s dedication to supporting specialty research while providing comprehensive and strategically aligned solutions for Sponsors and CROs. … Read more
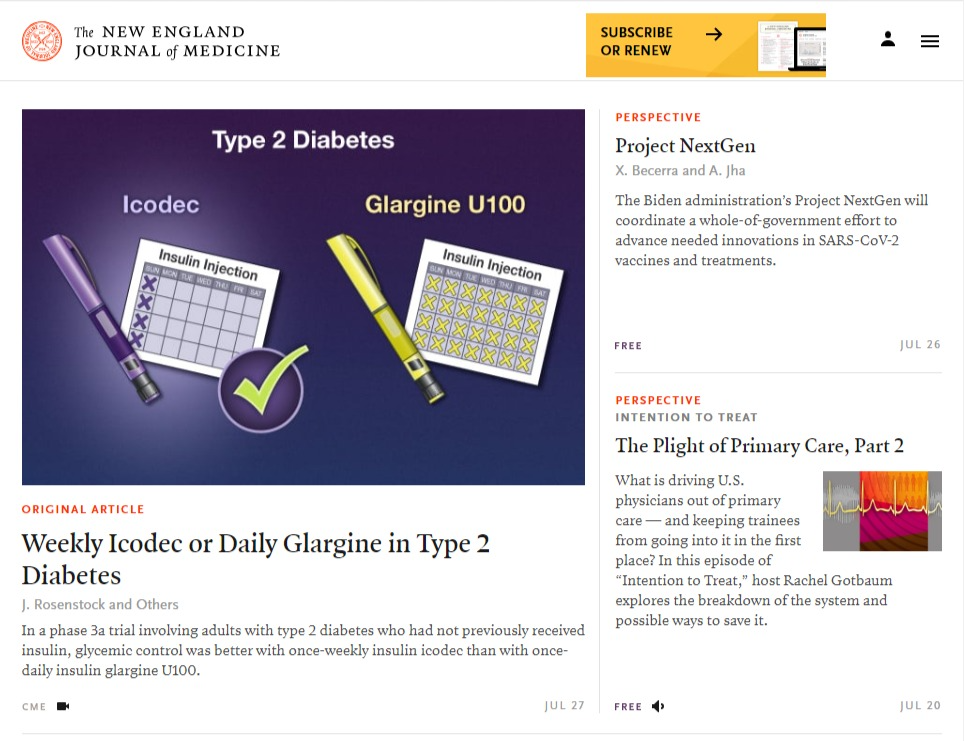
Julio Rosenstock, MD, has his work spotlighted as the feature article on the New England Journal of Medicine’s homepage. Serving as the lead investigator for Novo Nordisk’s ONWARDS 1 program, Dr. Rosenstock has made remarkable strides in exploring what could be the world’s first … Read more
For the team at Velocity Clinical Research in the United Kingdom, clinical trials are first and foremost about building relationships. “I’m really grateful to be involved in clinical trials, because it allows me to develop meaningful connections with people in a way that goes … Read more
Velocity Clinical Research (“Velocity”), the leading multi-specialty clinical sites business, and Privia Health (Nasdaq: PRVA), a physician enablement company serving more than 3,700 providers and 4.4 million patients, today announced a partnership to open embedded clinical research sites within Privia’s practice network. The partnership … Read more
With nearly three decades as a family physician and almost 25 years as a Principal Investigator (PI), Frank Eder, MD, believes two primary elements are critical for sustained success in clinical research. “You must have a true interest in the research side of it. … Read more
Julio Rosenstock, MD, and Juan Frias, MD, were featured prominently throughout the American Diabetes Association (ADA) 83rd Scientific Sessions. The prolific researchers also had findings simultaneously published in the New England Journal of Medicine and The Lancet. Dr. Rosenstock is the lead investigator for … Read more
The Velocity Clinical Research team in Los Angeles, CA, is tackling two of the biggest health threats to Americans today: obesity and diabetes. Better still, they’re making tremendous progress against these conditions that exact a terrible toll on quality of life and longevity. Consider … Read more
Learn more about Velocity's capabilities
Whether you’re ready to conduct a single-site study or a complex, high-volume trial, Velocity will ensure you have the right investigators, clinical staff, and patients for your research program.
Join a remarkable team doing remarkable work
Velocity careers offer competitive pay and benefits, and reward high performance with excellent opportunities.
Whether you're an industry veteran or are looking to take your first step into clinical research, we invite you to apply at Velocity.