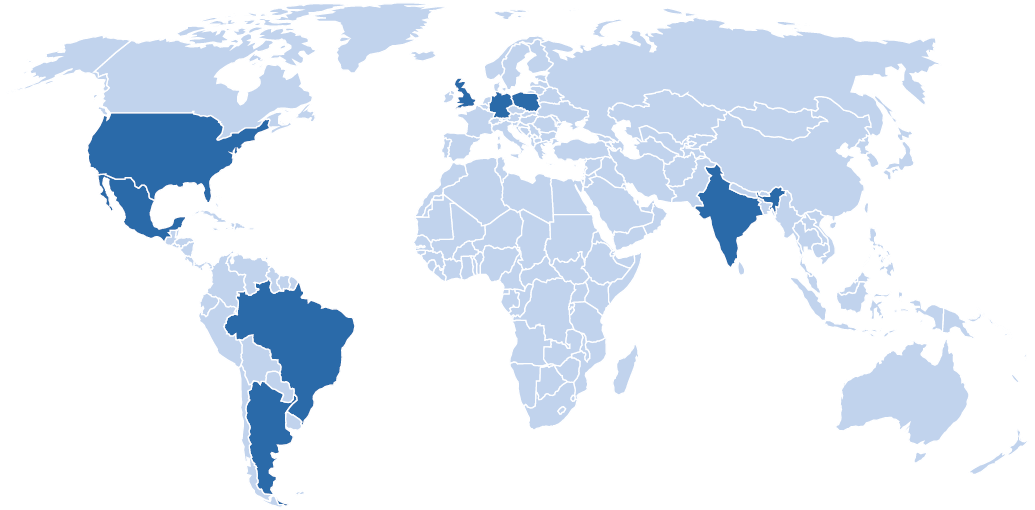
Velocity is expanding to Latin America, focusing on Brazil, Argentina, and Mexico
Velocity has appointed Renata Berardocco as Executive Vice President and Managing Director for LATAM. Renata has nearly two decades of experience in senior leadership roles and joins from Fortrea, where she led Clinical Operations Delivery and Clinical Projects.
Renata’s appointment signals Velocity’s ambition to further extend its global network. As LATAM MD, she will spearhead the identification, acquisition, and operations of new regional sites.
Paul Evans, Velocity’s President and Chief Executive, commented on the appointment: “Having established traction in Europe, LATAM is a natural next step for our growth-through-acquisition strategy. Renata brings a wealth of experience in building and managing multisite networks and shares our vision for the region. Renata will drive this next stage of Velocity’s growth, focusing primarily on Brazil, Argentina, and Mexico, which account for roughly 70% of the LATAM population.”
Renata’s appointment brings a valuable perspective and deep expertise. During her time at Novartis, she gained experience in a variety of areas, including governmental sales. She played a leading role in establishing a Brazilian presence for Oncopartners and set up the Brazilian clinical research department for Stiefel, which GSK later acquired. Most recently, she drove the expansion of Covance’s (now Fortrea) LATAM operations, where the local team grew from 300 personnel to 800.
Renata Berardocco, LATAM Executive Vice President and Managing Director, said: “I understand all too well the clinical trial process and how critical patient recruitment is. Velocity’s fast growth and commitment to technology make it a formidable force in clinical research. Multisite networks offer key benefits, from faster patient enrollment and consistent data collection to enhanced quality control and reduced variability — ultimately improving data security, trial efficiency, and patient outcomes."



Experienced Investigators, Specialists, and Research Staff
Therapeutic expertise
- Allergy/Immunology
- Cardiology
- Consumer Health
- Dermatology
- Endocrinology/Metabolic Disorders
- Family Practice
- Gastroenterology
- Infectious Disease
- Internal Medicine
- MASH/MASLD
- Medical Devices
- Nephrology
- Neurology
- Obesity
- OB-GYN
- Oncology
- Ophthalmology
- Orthopedics
- Pain Management
- Plastics, Reconstructives
- Psychiatry
- Pulmonary and Respiratory
- Rheumatology
- Sleep Medicine
- Smoking Cessation; Drug and Alcohol Abuse
- Urology
- Vaccine
Specialty populations
- Fetal and Newborn Health
- Elderly
- Healthy Normal Subjects
- Pediatrics, Adolescents
- Women's Health
Specialized trial support
- Biosimilars
- Combination products
- Devices
- Decentralized
- Diagnostics
- Hospital
- Interventional
- Observational
- Overnight